IEC 60601-1 and Power Supplies for Medical Devices
In this article, learn about medical devices' associated risk guidelines, the means of protection mandatory for their use, their specific use environments, and the electromagnetic compatibility issues that should be considered when designing their power supplies.
A rise in demand for medical devices supporting telehealth has led to a need for more IEC 60601-1 compliant power supplies. Image courtesy of CUI
The rise of more advanced home health devices, along with the growth of telecare as a result of COVID-19, has led to a proliferation of increasingly complex medical devices in a variety of settings. With these diverse settings and use cases of medical devices, designers are faced with a fundamental problem that must inform the design process from the ground up: how does one design medical devices (and, in particular, the power supplies for those devices) so that they can be used safely and appropriately?
This article explains the risk guidelines that apply to medical devices that are within a patient’s vicinity, the means of protection that are mandated for different types of medical devices, the intended use environments for these devices, and electromagnetic compatibility (EMC) issues that should be accounted for when designing power supplies for medical devices in any setting. These guidelines are defined by IEC 60601-1 and its collateral standard, IEC 60601-1-2.
Reviewing IEC 60601-1 Standards & Collaterals
The original IEC 60601-1 standard (also known as EN 60601-1 in Europe and CSA 60601-1 in Canada) has been in existence for 40 years. There are two different standards of importance (as related to medical power supplies) that this article focuses on: the first is edition 3.1 of the base standard, IEC 60601-1 Medical Electrical Equipment - Part 1. The second is the collateral standard IEC 60601-1-2 Electromagnetic Disturbances, which is in its 4th edition.
Type B, BF, and CF Medical Equipment Requirements
The 2nd edition of IEC 60601-1 provides the requirements associated with devices within the “patient vicinity,” defined as a 6-foot radius of the patient, as illustrated in Figure 2. There are three classifications related to patient focus: Type B, Type BF, and Type CF. Each classification comes with its own parameters that will affect the design approach for the power supplies for each type of medical equipment.
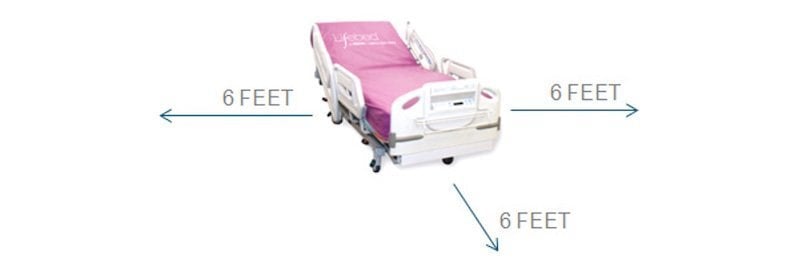
The patient vicinity is defined as a circle with a 6’ radius surrounding the patient. Image courtesy of CUI
- Type B (body) equipment is designed to operate within a patient's vicinity but without making actual contact with the patient. This classification applies to devices such as x-ray machines, MRI scanners, hospital beds, and LED operating lighting. Type B devices require 1500 Vac isolation, 2.5 mm creepage (i.e., the shortest distance to another conductor along the insulating surface), and basic insulation.
- Type BF (body floating) equipment does make physical contact with the patient and includes thermometers, blood pressure monitors, and ultrasound equipment. These devices require 3000 Vac isolation, 5 mm creepage, and double insulation.
- Type CF (cardiac floating) equipment is designed to make physical contact with the heart. This classification includes dialysis machines and defibrillators. For type CF devices, the requirements are 4000 Vac, 8 mm creepage, and double insulation.
Whether used in home or on-site at medical facilities, the design and use of medical devices can be profoundly constrained by its vicinity to the operator of the device and the patient.
Means of Protection (MOP)
A related - but distinct - consideration involves the Means of Protection (MOP) that are afforded to operators and patients of medical devices. First, a quick review of key abbreviations:
- MOP – Means of Protection, includes MOOP and MOPP
- MOOP – Means of Operator Protection
- MOPP – Means of Patient Protection
MOP classifications and their requirements, per IEC 60601-1 3rd edition, are as follows:
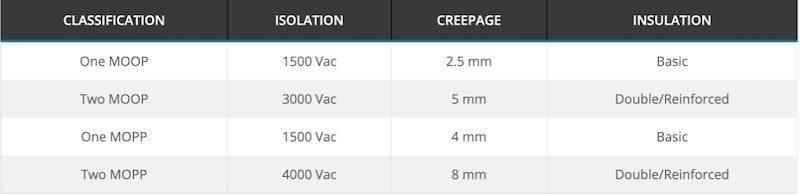
Requirements by Classification. Image courtesy of CUI
As an example, recall that Type B devices require 1500 Vac isolation, 2.5 mm creepage, and basic insulation. However, under the IEC 60601-1 3rd ed, this would only qualify as a 1 x MOOP certification. To achieve 1 x MOPP, 4 mm creepage is required. To be 2 x MOPP-certified, double insulation with 4000 Vac isolation and 8 mm creepage would be needed. Understanding what MOP classification you need your devices to conform should directly inform each step of your design lifecycle.
EMC Issues and Intended Use Environments
Medical devices are not just found in hospital settings: the COVID-19 pandemic, among other contributing factors, has directly led to an increased use of home health devices that will often be surrounded by devices that can lead to electromagnetic interference, including laptops, tablets, smartphones, and other devices that use Wi-Fi, Bluetooth, and cellular networks. It is worth noting that COVID-19 has significantly increased the demand for power supplies in telehealth and led to some new challenges.
According to IEC 60601-1-2, designers must consider the electromagnetic compatibility of their medical devices, which is defined as “the basic safety and essential performance of medical equipment and systems in the presence of electromagnetic disturbances and electromagnetic disturbances emitted by that equipment and systems.”
The IEC standard considers the intended use when setting EMC requirements. In addition to this, the collateral standard IEC 60601-1-11 covers the home healthcare environment and applies additional safety and use requirements on top of the base standard.
When setting EMC requirements for a medical product (including power supplies), the environment where the product will be used is considered. There are three types of intended use environments:
- Professional healthcare facilities
- Home healthcare
- “Special” environments
Professional healthcare facilities include intensive care units, hospitals, and dental offices -- all of which have attending medical staff. Special environments could be areas where high-power medical equipment is being used, such as radiotherapy equipment, or areas where high levels of electromagnetic disturbance are possible.
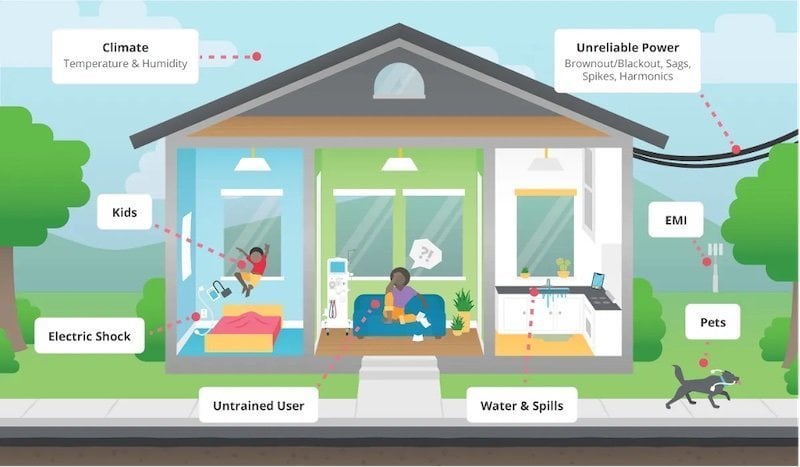
There are a variety of potential hazards in a home health environment. Image courtesy of CUI
However, home health environments are not the same as professional healthcare facilities because the medical equipment must tolerate a poor electrical supply, is likely to be used by non-specialists, and will often be operated in an uncontrolled environment (as illustrated in Figure 4). Home medical devices can include glucose monitors, blood pressure monitors, thermometers, pulsometers, home dialysis machines, and similar devices -- all of which can also be a part of telehealth monitoring and treatment.
For example, if a device is intended for use in a home health environment, it would need to meet the base IEC 60601-1 edition 3.1, the EMC requirements of IEC 60601-1-2 edition 4 based on the intended environment of the home, and the added safety requirements of IEC 60601-1-11 for home medical.
CUI’s New Compliant Medical Power Supplies
As we have seen in this article, power supply requirements must be met to ensure safety for both users and patients. However, navigating IEC requirements related to medical devices is often confusing for those unfamiliar with them. In addition, demonstrating compliance with the correct standards can be time-consuming and potentially extend the time to market for new products.
One solution to a compliant power supply is designing pre-certified power sub-systems. CUI has a range of AC-DC power supplies designed for use in the medical field that are certified to the most recent edition of the international safety standard IEC 60601-1, edition 3.1, as well as the 4th edition EMC requirements, and solutions that are certified for 2 x MOPP applications.
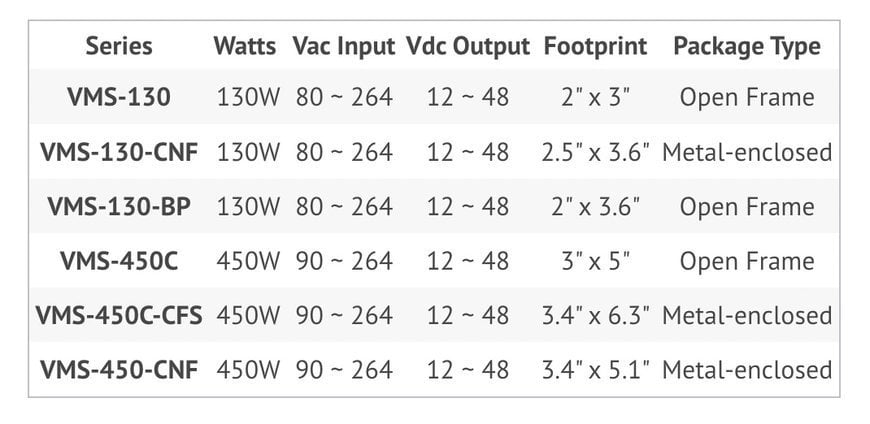
Table 1. CUI’s medical-grade power supplies are all 2 X MOPP-certified, and they include active power factor correction and operate at high efficiency. They are well-suited for medical and dental applications.
The new medical power supply lineup includes the VMS-130 series, which are open frame 130W, 80 ~ 264 Vac input, 12 ~ 48 Vdc output option power supply designed for medical and dental applications. These chassis mount VMS-130 lines that offer active power factor correction and efficiency up to 94%, all within an industry-standard footprint of 2” x 3”. Product lines within the VMS-130 series include the VMS-130-CNF series, which is metal-enclosed, and the VMS-130-BP series, an open frame version with baseplate cooling.
CUI also has the 450W VMS-450C chassis mount series, which shares the power factor correction and efficiency of the VMS-130 series but with 90 ~ 264 Vac input, 12 ~ 48 Vdc output options, and 3” x 5” footprint. This product line, certified to the medical 60601-1 3rd edition safety standard for 2 x MOPP applications, includes the VMS-450C-CFS metal-enclosed series and the VMS-450C-CNF series, which also provides a power good signal and remote sense.
Summary
Medical devices, including home health and telehealth devices, must comply with a rigorous set of international safety standards. IEC 60601-1 and its collateral standard, IEC 60601-1-2, are the primary documents that govern requirements for power supplies in medical devices. For those integrating medical-grade power supplies into their designs, CUI offers multiple IEC-compliant offerings that can streamline the design process.
www.cuidevices.com

